WMozzies WhiMSICAL CART-WHEEL database project
 WhiMSICAL database flyer DOWNLOAD <—– Select “DOWNLOAD” for copy of WhiMSICAL database flyer
WhiMSICAL database flyer DOWNLOAD <—– Select “DOWNLOAD” for copy of WhiMSICAL database flyer
 Information on how to JOIN <—- Select “JOIN” for Participant Information, How to Provide your Consent and Join CART-WHEEL
Information on how to JOIN <—- Select “JOIN” for Participant Information, How to Provide your Consent and Join CART-WHEEL
WM patients globally are invited to contribute their details to the WhiMSICAL (Waldenström’s Macroglobulinemia Study In CART-WheeL) database, and print out your own summary for personal use. Data entered by / for WM patients will contribute to research on our disease. The database is designed to address some of the barriers facing effective research into our rare cancer. The aim is to gain a better, understanding of WM symptoms and correlation to pathology results, family history and genetics. The research also covers triggers to commence therapy, different treatments, their efficacy and tolerance, as well as disparities in treatment access within countries and internationally. Data from WM patients provided in the pilot launch of WhiMSICAL has already been presented at international conferences. This is an opportunity for WM patients to drive international research into their disease.
Information follows on:
- Why join WhiMSICAL Database
- Features of WhiMSICAL
- History of WhiMSICAL
- How WhiMSICAL works
- How to take part in WhiMSICAL
- Consent for use of your de-identified data by Research Investigators
- Tips on getting started with WhiMSICAL
- Enquiries and support
- Consent form completion
- Examples of WhiMSICAL Database Questionnaire completion
1. Why Join WhiMSICAL?
Joining WhiMSICAL will help current and future WM patients around the world. WhiMSICAL is the first patient- and medical researcher-led global patient database for Waldenström’s Macroglobulinemia. WhiMSICAL captures patient data for WM patients globally. WhiMSICAL uses the same analytical framework used for many other rare diseases.
Efforts to create a global database have been conducted in the past. However, they have not been as extensive or as scientifically valid as WhiMSICAL promises to be. While major medical centres have records of their patients’ medical histories, they do not necessarily share those data with each other. WhiMSICAL is the only database thus far that collects patient data from around the world in one centralized location.
WhiMSICAL is heading towards big data analytics. The goal is to have a database for examining large and varied data sets – i.e., big data – to uncover hidden patterns, unknown correlations, trends, patient preferences and other useful information that can help medical researchers make more-informed decisions. We need to get a sufficient number of WM patients to enter their data. Then we need to compare it to information at medical institutions to make sure they correlate. Then, we can use those data to inform our understanding and to help frame further research needs for insights into WM.
2. Features of WhiMSICAL
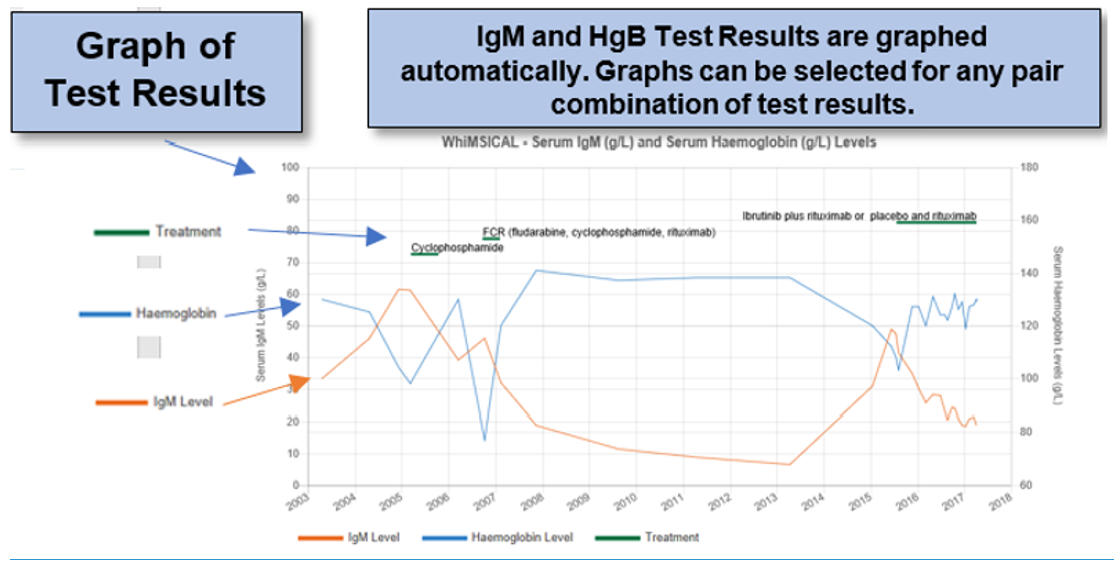
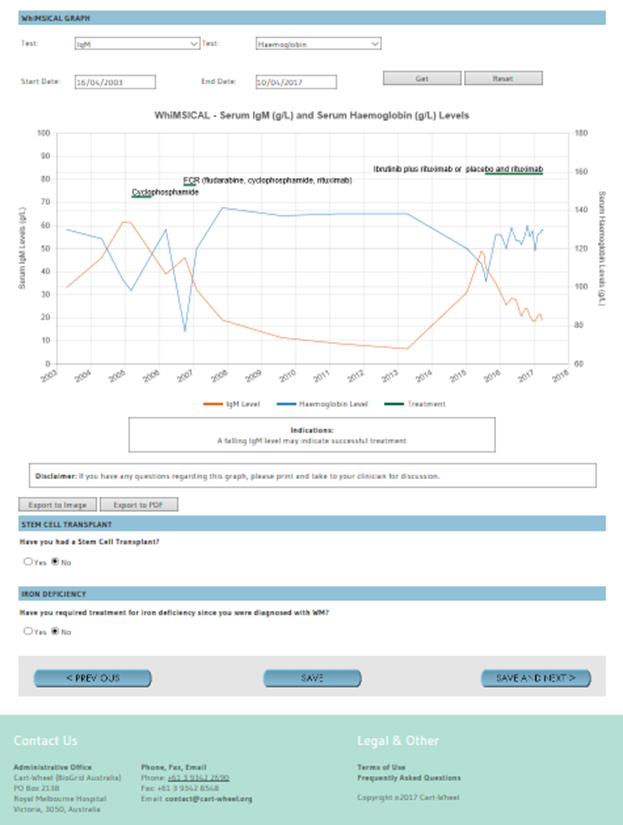
- A personal summary of disease experience, including graphing of your Haemoglobin and IgM levels to use as a personal profile and for use in consultations with your physicians, with capacity for ongoing revision with future entries
- Authorised clinician researchers gain access to de-identified data for a better understanding of Waldenström’s macroglobulinemia and for improved treatments for patients, both globally or for their own country.
- Access to data is solely to advance the research and information priorities of the investigators and patients and carers who enter the data. While the database is approved by the Ethics Committees of Royal Melbourne and Concord Repatriation General Hospitals in Australia. WhiMSICAL is a global research project, supported by the IWMF.
- A robust proven processing platform which securely holds medical data on over a million patients
- A powerful analytical tool to support ease of use by Investigators
- A potential for “Big-Data”
3. History of WhiMSICAL
The WhiMSICAL Database was launched in Australia in June 2016 as Project 100 and is now successfully established. Participants have entered their data into Australia’s CART-WHEEL.org (Centre for Analysis of Rare Tumors) and the de-identified data is analysed by the investigators. CART-WHEEL is an ethically approved WM-specific patient database utilising the BioGrid Australia secure database platform for rare cancers. BioGrid Australia is a well-established web-based, real-time, data-sharing platform for collaborative, translational medical research linking de-identified, ethically-approved data across institutions and jurisdictions. Following Australian promotion in 2016, the feasibility of WhiMSICAL was confirmed with 66 patients recruited. In 2017 pathology test result graphing and online consent were implemented.
The IWMF is now leading the global roll out of WhiMSICAL and encouraging English speaking global patient to participate.
An ASH200+ goal for recruitment of at least 200 patients by July has been set. We need YOUR contribution to make that first 200. This will support submitting an abstract to ASH 2017 demonstrating the global feasibility of WhiMSICAL.
A significant increase in WhiMSICAL data is anticipated in coming years with ongoing prospective patient data updates and global expansion. The breadth of information gathered will expand WM knowledge of the range treatment presentations and treatments. This will complement the depth of data derived from clinical trials and site-based registries. Demonstration of any treatment disparities, coupled with information regarding treatment efficacy may facilitate novel therapies
The WhiMSICAL database was presented at:
- 9th International Doctor-Patient Forum on WM in Amsterdam on, 9 October 2016 – IWMF President Carl Harrington at IWWM9 hosted a special briefing on the WhiMSICAL database for International WM leaders from US, UK, Netherlands, Germany and Greece clinicians generating strong interest and support.
- Meeting at the Melbourne Convention and Exhibition Centre on 15 November – Dr Treon spoke at meeting and conferred with WhiMSICAL Principal Investigators and “is excited to support WhiMSICAL”
4. How WhiMSICAL works
WhiMSICAL uses a privacy-protected, internet-based questionnaire. WM patients complete this questionnaire on the CART-WHEEL.org website. They have control over personal details by providing consent to different uses of entered data. A correlation study of patient-entered data has demonstrated a 75% concordance with hospital databases.
The WhiMSICAL CART-WHEEL questionnaire covers:
o Patient demographics
o Disease specifics: staging, chronology of symptoms, IgM levels, full blood count information, etc.
o Treatments, how accessed, and treatment side-effects
o Personal and family medical history
For individual WM patients, WhiMSICAL provides a summary (profile) of questions and responses. This is handy as an individual’s personal record and for review with their physician. Test results are also presented graphically. The graphs show trend lines for each of the Pathology results entered and include details of treatments received. Graphs may be tailored for any combination of test results and period desired.
5. How to take part in WhiMSICAL
Register (Create a CART-WHEEL account, https://www.cart-wheel.org/)
The setup of a CART-WHEEL account includes creating a username and a password to use to log in to the questionnaire. This gives an option to save information at any time and to log in later to update the information.
Complete the questionnaire
It will be useful before you start the questionnaire to have:
- a copy of your pathology report
- information about the hospitals or medical centres where you have been treated
- the names of the treatments that you have received
If you do not have all the required information immediately available, don’t worry, you can update your answers to those questions at a later date.
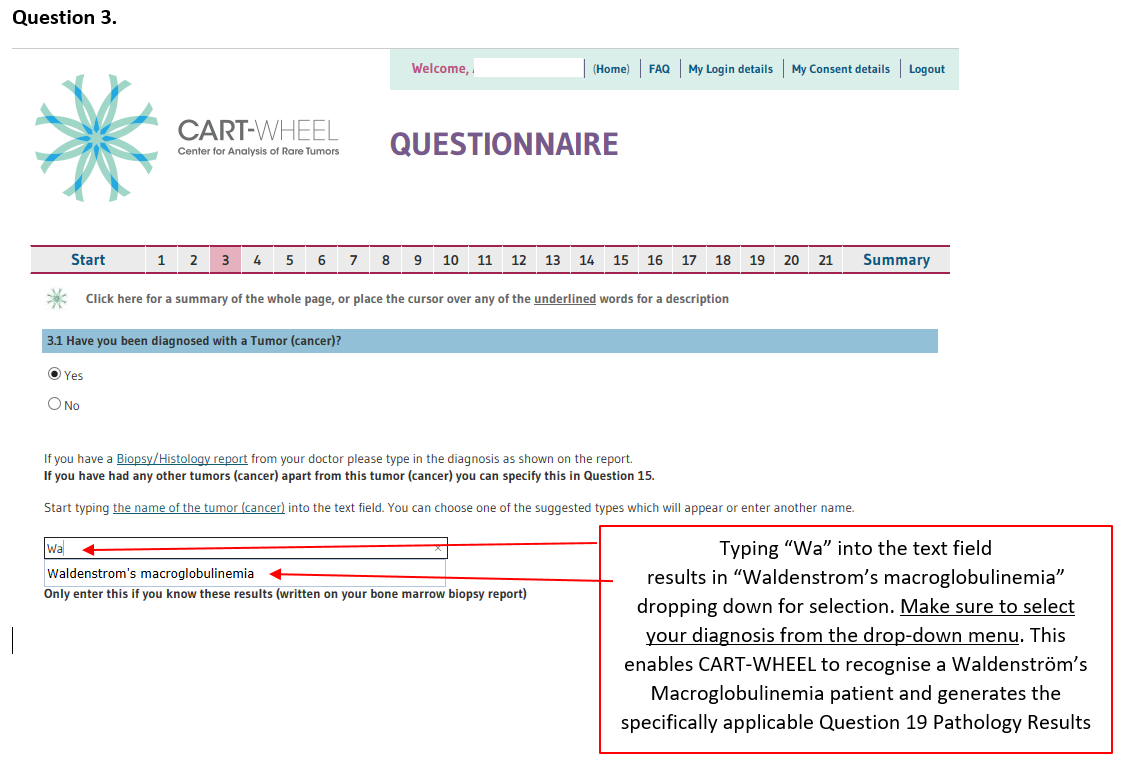
Your information will be entered via a secure connection and cannot be seen by anyone else. The WhiMSICAL database, is automatically generated when you enter Waldenström’s or lymphoplasmacytic lymphoma into your diagnosis in Question 3. Make sure you select your diagnosis from the drop-down menu (See below – 10. Example of WhiMSICAL database Questionnaire completion, Question 3.).
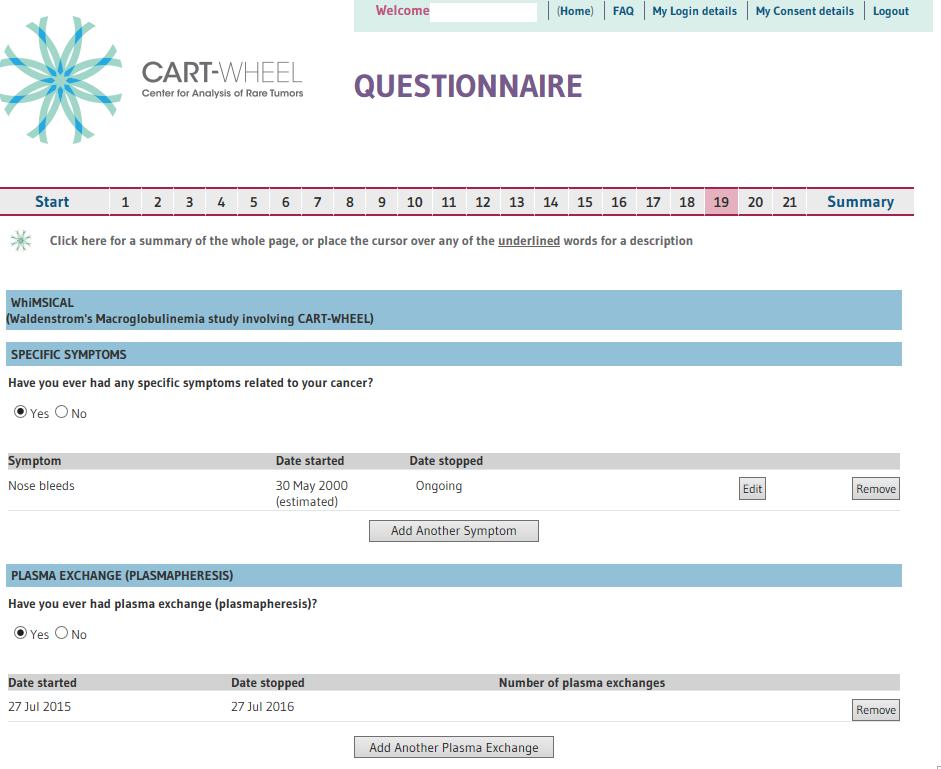
At the end of the questionnaire you will be able to print out a summary of questions and your responses for your record. (See below – 10. Example of WhiMSICAL database, Question 19 content.).
Entering the data requires WM patients to spend anywhere from half an hour to a few hours depending upon for how long they have had WM and how many treatments they have had. It is suggested you start by entering only readily available details. Login again later to enter details such as test results and BMB details. The time spent in recording your details is valuable for WM research and well worth the effort. Entry of pathology test results is most important, especially those around the time of diagnosis, before and after treatment and the most recent.
Complete the Consent Form (Without a completed consent form, the information provided cannot be used)
Completing your Consent Form confirms your permission for use of the entered information for the purposes of research. This is an important requirement of the Human Research Ethics Committee that protects your rights and confirms that you understand the purpose of this research project.
Submit the Consent Form to CART-WHEEL online.
Once you are logged into the Questionnaire, select ‘My Consent details’ in the top right of your screen. Select the ‘Add Consent’ button. This will provide on screen the relevant information to enable you to consent to your participation in CART-WHEEL.
6. Consent for use of your de-identified data by Research Investigators
Details recorded cannot be used for research until formal consent is given. The privacy and confidentiality of patient details in WhiMSICAL are safeguarded globally by strict ethical, scientific and legal standards. The CART-WHEEL project has been granted ethical approval by Melbourne Health Human Research Ethics Committee (HREC) based at The Royal Melbourne Hospital, Australia. This means that only with your consent, can your information be used to further cancer research.
In the Consent process, participants are given very detailed information about the project. All the procedures involved in this project are explained before you decide whether or not to take part in it. Once you understand what the project is about and if you agree to take part in it, you will be asked to provide your consent by completing the Consent Form. By completing and submitting the Consent Form, you indicate that you understand the information and that you give your consent to participate in the project.
7. Tips on getting started with WhiMSICAL
- Note throughout that CART-WHEEL uses the word “tumor” to refer to cancer of any part of the body, including blood and lymph glands
- Start with an easy small step and come back later to do more.
- In the first instance enter details for Questions 1-3 with demographics (age, location), date of diagnosis and then complete Consent.
- See separate tips for Question 3 Diagnosis and for Consent
- Give priority in data entry after entering Questions 1-3 data and Consent to:
- Question 9 Your Treatments
- Question 19.1 Your WM symptoms
- Question 19.5 Your Pathology results particularly your IgM and hemoglobin details at diagnosis, at start and completion of each treatment course and your most current data
- Note in Pathology test results in Question 19:
- Abnormal or Paraprotein Band (M-spike) in WhiMSICAL is called Serum Paraprotein Level in Question 19.
- Beta 2-microglobulin test is not done as frequently as others. It is often part of a panel used to determine prognosis and treatment. Beta 2 globulin is very different to Beta 2-microglobulin.
- Then as it suits you slowly work through the rest of Questions later
- .
Joining WhiMSICAL and spending a few hours entering your details will help current and future WM patients around the world. WhiMSICAL database will help us get closer to a cure and benefit WMers and WM researchers across the globe. Consider it your personal scientific contribution to conquering WM.
8. Enquiries and support
General information – IWMF and WMozzies: whimsical@iwmf.com
Support from WM patients using WhiMSICAL: whimsical@iwmf.com
Technical support and user problems: WhiMSICAL CART-WHEEL: contact@cart-wheel.org
Frequently Asked Questions http://www.wmozzies.com.au/index.php/whimsical/whimsical-frequently-asked-questions/
9. Consent form completion
The consent form is necessarily comprehensive to ensure the privacy and confidentiality of patient details. It covers:
- Introduction and background in pages 1 to 4
- BioGrid Australia on page 5
- Questionnaire completion on page 6
- Levels of consent on page 7
- Information contained in WhiMSICAL CART-WHEEL data base in pages 8 to 11
- How privacy is protected in pages 12 to 13
- Benefits, risks, further information, problems, voluntary participation in pages 14 and 15
- Ethical guidelines on page 16
- Consent permission on page 17
- Consent summary confirmation on pages 19 and 20
Suggested Tips for WM patients to assist completion of form follow:
- CART-WHEEL uses the word “tumor” to refer to cancer of any part of the body, including blood and lymph glands.
- Choose Online option for Consent form as it is easier and quicker
- Consent to all 5 options on page 7 to ensure the maximum benefit for research purposes from data provided
- The sections of the consent form on pages 8 to 10 regarding tissue sample specimens and blood samples stored in tissue banks are not relevant to the WhiMSICAL CART-WHEEL Database
- For assistance and any clarification send message to whimsical@iwmf.com
10. Examples of WhiMSICAL Database Questionnaire completion
Question 19.