WhiMSICAL Registry –
A global WM patient-derived data registry capturing treatment, quality of life and COVID-19 data

A global WM patient-derived data registry capturing treatment, quality of life and COVID-19 data
- WM patients globally are invited to contribute their details to the WhiMSICAL (Waldenström’s Macroglobulinemia Study In CART-WheeL) Registry.
- Patients anywhere in the world can join, consent and enter their data to contribute to WM research.
- Early results of WhiMSICAL’s patient-entered data have already been published in a leading US hematology journal- American Journal of Hematology
- WhiMSICAL Registry captures how effective different treatments are and how they can impact quality of life
- It is a flexible, secure platform, with COVID-19 impact and vaccine questions recently added
- Access to data is solely to advance the research and information priorities of the investigators and patients and carers who enter the data.
- The Registry is approved by the US Western Institutional Review Board (WCG IRB) and by the Australian Ethics Committees of Royal Melbourne and Concord Repatriation General Hospitals
WhiMSICAL aims to develop a global platform for obtaining real world “big data” in WM with ongoing recruitment and patient data entry to:
- Cover a research void of insufficient well-founded evidence, as WM is a rare disease with limited phase III clinical trial data
- Increase knowledge of presentations and treatment outcomes of WM patients
- Map patient reported outcomes (PROs) to therapies
- Generate hypotheses to advance WM research
- Facilitate patient preference research
Information follows on how WhiMSICAL is heading towards big data analytics to uncover hidden patterns, unknown correlations, trends and patient preferences, covering:
- Features of WhiMSICAL
- History of WhiMSICAL
- How WhiMSICAL works
- How to take part in WhiMSICAL.
- Enquiries and support
- WhiMSICAL Registry flyer
1. Features of IWMF WhiMSICAL Registry include:
- WhiMSICAL Registry is a global research project, supported by the IWMF.
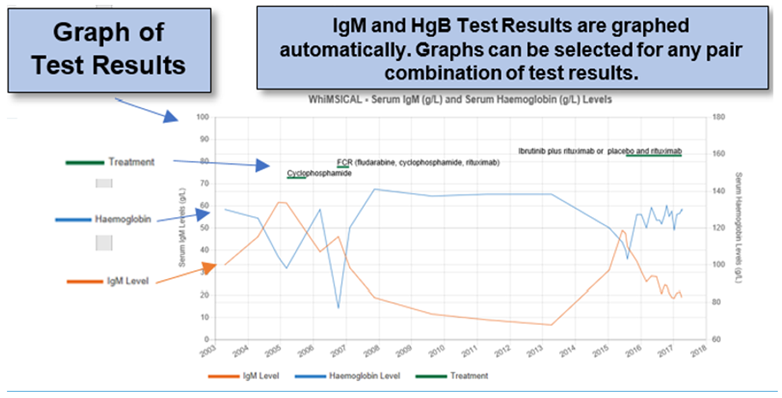
- A personal summary of disease experience, including graphing of your Hemoglobin and IgM levels to use as a personal profile and for use in consultations with your physicians
- Capacity for ongoing revision with future entries
- Authorised clinician researchers gain access to de-identified data for a better understanding of Waldenström’s macroglobulinemia and for improved treatments for patients, both globally or for their own country.
- Access to data is solely to advance the research and information priorities of the investigators and patients and carers who enter the data.
- The Registry is approved by Western Institutional Review Board in the US and the Ethics Committees of Royal Melbourne and Concord Repatriation General Hospitals in Australia.
- A robust proven processing platform which securely holds medical data on over a million patients
- A powerful analytical tool to support ease of use by Investigators
- A potential for “Big-Data” comparisons with information at hospital institutions to aid informed decisions by medical researchers
2. History of WhiMSICAL
Initial recruitment efforts illustrated below were driven through messaging by WMozzies, to establish feasibility of the platform, resulting in 69 patients (16%) joining the study in 12 months (29% of study duration). Subsequent social media messaging by the IWMF to members globally led to a significant surge in the number of participants (A). Messaging regarding the addition of the Quality of Life questions EORTC QLQ-C30 to the questionnaire led a second surge in recruitment (B). COVID-19 questions were added in 2020-21.
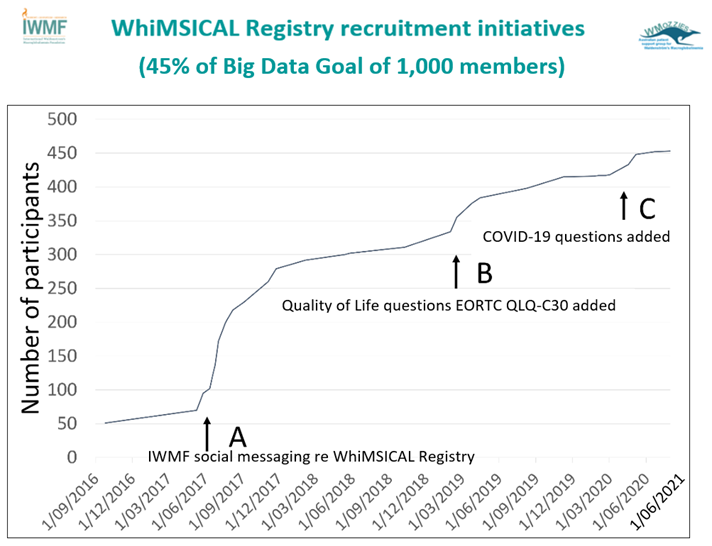
WhiMSICAL Registry 453 members from 19 different countries.
46% of members are from the US.

3. How WhiMSICAL works
WhiMSICAL uses a privacy-protected, internet-based questionnaire. WM patients complete this questionnaire on the CART-WHEEL.org website. They have control over personal details by providing consent to different uses of entered data. A Correlative study of WhiMSICAL patient-derived data with Lymphoma and Related Diseases Registry available data has confirmed an 80% agreement. There were high completion rates and good concordance with diagnosis and treatment data but higher variability with IgM data.
The WhiMSICAL CART-WHEEL questionnaire covers:
- Patient demographics
- Disease specifics: staging, chronology of symptoms, IgM levels, full blood count information, etc.
- Treatments, how accessed, and treatment side-effects
- Personal and family medical history
- Quality of Life (EORTC QLQ-C30) Patient Reported Outcomes (PROs)
- COVID-19 impact and vaccines
For individual WM patients, WhiMSICAL provides a summary (profile) of questions and responses. This is handy as an individual’s personal record and for review with their physician.
Test results and the overall Quality of Life scales are also presented graphically.
The graphs show trend lines for each of the entered Pathology results and include details of treatments received.
4. How to take part in WhiMSICAL
Register Create a CART-WHEEL account at https://www.cart-wheel.org/
The setup of a CART-WHEEL account includes creating a username and a password to use to log in to the questionnaire. This gives an option to save information at any time and to log in later to update the information.
Give Consent – Click on the “My Consent details” tab Data entered in Questionnaire is not available for Research until Consent is given.

5. Enquiries and support
General information – IWMF and WMozzies: whimsical@iwmf.com
Support from WM patients using WhiMSICAL: whimsical@iwmf.com
Technical support and user problems: WhiMSICAL CART-WHEEL: contact@cart-wheel.org
Frequently Asked Questions http://www.wmozzies.com.au/index.php/whimsical/whimsical-frequently-asked-questions/
WhiMSICAL Registry flyer





